NeoVista Epi-retinal Strontium 90 Treatment for Wet AMD
On January 16, 2007, Dr. Andrew Schachat, Vice Chair for Clinical Affairs, Vitreoretinal Department of the Cole Eye Institute, presented the first data on NeoVista’s Epi-Rad 90 Ophthalmic System at the Hawaiian Eye meeting (RHEM). The procedure involves the epi-retinal application of strontium 90 radiation for the treatment of wet AMD. A review of some of the early findings from his presentation are reported below.
Radiation treatment for AMD
NeoVista, Inc., of Fremont, Calif, released data demonstrating a potential benefit of treating wet AMD with radiation using the company's Epi-Rad 90 Ophthalmic System. Clinical data was presented from 2 separate feasibility studies that utilized the NeoVista product. The first study, involving a total of 24 patients, comprised 2 different doses of radiation (15 Gy and 24 Gy) delivered to the retina. The second study, which involved 20 patients, utilized a concomitant approach of 24 Gy radiation plus Avastin, where an injection of the drug was administered at the time of radiation treatment and an additional injection administered 30 days later. This is the approach the company plans to follow when it begins its pivotal 450-patient CABERNET trial this year. The company will utilize Lucentis instead of Avastin in this trial.
The Procedure and Early Results
The procedure involves performing a partial vitrectomy under local anesthesia. The delivery of the radiation takes between 3 and 5 minutes, depending on the calibration certification for the device being used, while the total procedure takes about approximately 40 minutes. The focal radiation penetration is about 3 mm into the choroid. The company claims that the ionizing radiation has a toxic effect on local pro-inflammatory and fibroblast cell populations, permanently disabling the proliferating CNV Cells.
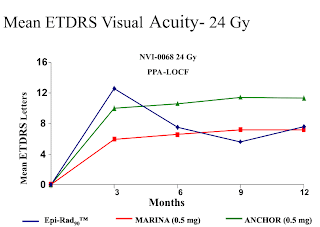
In the two feasibility studies, using radiation alone, the lower dosage test (15 Gy) was not impressive, showing a loss in visual acuity after 9 (-2.4 letters) and 12 months (-3.2 letters).The study utilizing a higher dosage of strontium 90 (24 Gy) had better results, showing an increase in visual acuity after 9 (+5.6 letters) and 12 months (+7.6 letters). This compared favorably with the MARINA study of Lucentis, which had an increase of +7.2 letters after 12 months.
However, the second study, which utilized a concomitant approach of 24 Gy radiation plus Avastin, where an injection of the drug was administered at the time of radiation treatment and an additional injection administered 30 days later, was even more impressive. After only 2 and 3 months followup, the 20 patients showed an increase in VA of +14.2 and +14.9 letters, beating the MARINA study results by a wide margin.

Eugene de Juan, Jr., MD, the Jean Kelly Stock Professor of Ophthalmology at the University of California San Francisco and the inventor of the NeoVista treatment approach, commented, "Although the follow-up period (3 months) is relatively short, the results observed from the concomitant trial are extremely encouraging. The percentage of patients who improved in visual acuity by greater than 3 lines was reported at 50%, which is far above the 34% that was reported in the Lucentis MARINA Study. Granted, the NeoVista sample size is much smaller than that garnered from MARINA, but the evidence does support a closer investigation of this concomitant approach."
"We continue to see vision stability, and in many cases, vision improvement after just one treatment," stated John Hendrick, President and CEO of NeoVista. "The potential impact of our technology will greatly benefit patients, physicians, and the overall health care system. We will soon begin our Pivotal Trial incorporating concomitant use of Lucentis and Epi-Rad 90 therapy. The encouraging results observed in our concomitant feasibility trial have us excited about the future of this approach."
CABERNET Trial Design
According to the company, the CABERNET trial will involve 450 patients, with 300 receiving the 24 Gy dosage of strontium 90 plus Lucentis, and the control arm of 150 patients will receive Lucentis only. The study, to begin later this year, is scheduled to be held at 30 sites worldwide, 20 in the U.S. and 10 OUS. We will keep you informed on more details of the new trial as we learn them.
Questions & Answers
After reviewing Dr. Schachat’s Powerpoint presentation, I asked several questions of the company’s Vice President of Marketing & Sales, Tony Moses. Here are my questions and his responses:
1. Isn't even a partial vitrectomy more invasive than just the injection used for Lucentis/Avastin?
Any vitrectomy is naturally more invasive than an injection. What is not known, and what concerns many physicians is the potential outcome of numerous intraocular injections over time.
2. The higher dose appears to work better than the lower dose (duh!), but with a higher percentage of adverse results. Are there other dangers from the higher radiation dosage to the retina?
The only noticeable adverse effects in either group is the number of cataracts caused by our procedure, which is still in line with that reported by Lucentis in their MARINA data. To date (and we now go out to 18 months with some patients) there are no other significant adverse effects to report. Physicians are still concerned with the potential of causing radiation retinopathy, although we have seen no such cases yet.
3. How often does the treatment have to be repeated? (I don't think I saw that in looking quickly at the presentation.)
5. Do you have an estimate of the cost? How will it compare, say, to a year's treatment with Avastin?
We estimate that our procedure will cost roughly $6,000 over a two year period, exclusive of the drug cost. To compare it to Avastin will be difficult since we have no data on the number of annual injections required to temper the disease. If we use Phil Rosenfeld's data from PrONTO, the number is roughly 6 per year. At $50 per injection (typically paid by the patient) plus the cost of injecting the drug ($200 for 2007) the Avastin cost will total ~$1,500 per year, not counting the cost of all exams. Please keep in mind that even though the patient may not require monthly injections, they must still be seen every 4 to 6 weeks to confirm disease stability. And, since the drug is not curing the disease, patients will likely have to continue this approach on an ongoing basis. This is one of our potential benefits - less burden on both the patient and the physician.



1 Comments:
This looks like promising work. Thank you for the advance information and please let us know when the trial begins recruitment.
Post a Comment
<< Home